Did not receive verification mail? Please confirm whether the mailbox is correct or not Re send mail

Vapor
- 2020-02-28 11:22:15
5th China Pharma IP Summit 2020 will be held on Oct 14th to 17th
The Development of U.S. China Trade Deal/Patent Law Amendment, Implementation Rules and Laws for Patent Linkage and Patent Term Extension
Challenges and Opportunities Under China Ongoing Generics Consistency Evaluation, New IP Landscape for Generics and Innovators
Patent Examination Standards & Differences and Determination for Patent Infringement in China, EU and US
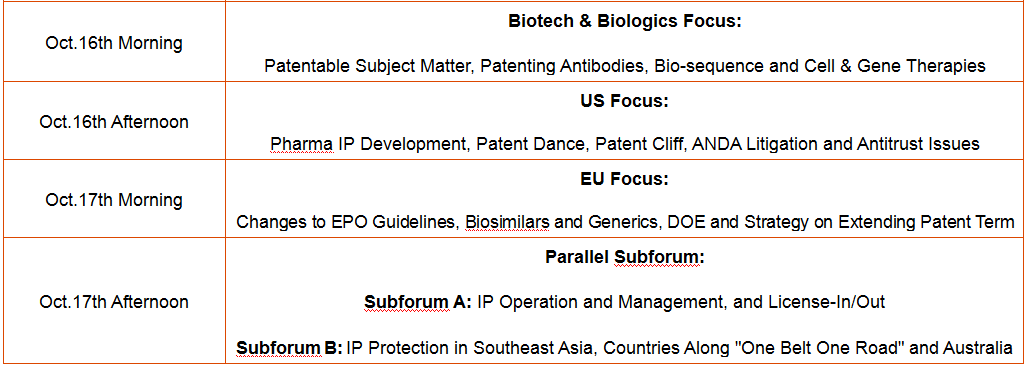
Pharma IP System, Recent Developments, Key Decisions and Latest Challenges in Japan, South Korea, India, Southeast Asia, Countries Along "One Belt One Road" and Australia
Generics and Biosimilars in China, EU and US: IP Landscape, Regulatory Issues, Patent Cliff and Market Entry
Recent Trends and Effect of ANDA Litigation and Biosimilar Litigation in US
Applicable Scope and Comparison of SPC in EU and PTE in US, Lifecycle Management and Patent Portfolio Development
Patenting Antibodies, Cell & Gene Therapies and Cutting Edge Technologies: Patentable Subject Matter, Special Requirements in Patent Application and Examination Proceedings
Medical Use Patent Developments: Comparison of Examination and Granting Rules in China, EU and US
Patent Licensing: Considerations, Negotiation, Agreements Drafting and Pitfalls
65+ Eminent speakers sharing their Insights and practices in different jurisdictions
350+ Domestic and foreign drugmakers (Including scientific research institutions
90+ Leading law firms and IP agencies from local and abroad
750-1000+ In-house Pharma IP counsel, patent prosecutors and litigators, government officials and policy experts
20+ Hours networking opportunities during this 3 and half day conference(Tea break, Three days lunch and Two cocktails for all participants)

In recent years, China has taken many powerful reform measures to strengthen the protection of intellectual property rights, create a protective environment for innovation in the pharmaceutical industry, accelerate the transformation and upgrading of generic drug companies, balance innovation, competition and prescription drug affordability. According to the signed phase one deal of China-US Economic and Trade agreement on January 15, 2020. Some patent provisions related to pharma intellectual property including patent linkage, patent term extension and post-filling supplemental data which are offering very positive signals to the industry peers and adding further confusion on how and when these provisions can be implemented in China through laws or regulations. No matter how the final implementation goes, it will have a profound impact on the integration of China's pharmaceutical market into the global innovation system.
As the second largest pharmaceutical industry in the world and China continue to widen the door for foreign drugmakers to get market access. Meanwhile, Domestic players are also stepping up their activities in overseas country. In this process, it is particularly important for foreign and domestics players to navigate the changing pharma IP and regulatory landscape in China and the rest of the word.
Against the backdrop, the 5th China Pharma IP Summit 2020 will once again bring together leading in-house IP counsel, patent prosecutors and litigators, government officials and policy experts from around the world who will share their insights on the latest IP strategies for thriving at a time when regulatory and IP landscape changes are significantly impacting your business models.
This event truly is the largest of its kind to be held in Shanghai China from Oct 14th to 17th , and we will be thrilled to have you join us to reap the benefits of the handpicked, high-level and well-informed presenters, dedicated networking breaks and stimulating interactive sessions taking place over this jam-packed three-day event and Plus few Pre-Conference workshops.
Get Insights to Address:
CPIPS 2020 Conference Structure :

CPIPS 2020 Highlights:The Most Compelling Reasons to Attend!
Source:iprdaily.com
Editor:Vapor
- I also said the two sentence
- Also you can enter 140words






