Did not receive verification mail? Please confirm whether the mailbox is correct or not Re send mail

Vapor
- 2019-06-03 16:38:19
4th China Pharma IP Summit 2019
Following the latest development of China Patent Law Amendment, IP Enforcement, Patent Linkage, Patent Term Extension Reform, and related Drug Regulatory Updates in China
Addressing Challenges and Uncertainties under BREXIT, SPC, Unitary Patent & UPC in Europe
Understanding the differences and updated requirements of examination standards, patent invalidation, patent litigation in China, US, EU, Japan and other key regions
Learning IP management and practical experiences from Global and China leading pharma companies
Meeting your clients/partners face to face and interact with 450+ industry peers
The Present Situation & Trends of Patent Examination and Hotspot Issues in Reexamination and Invalidation Cases in China
IP Enforcement and Protection in China
Interpretation: The Latest Drug Regulatory Policies and Implementing Details from National Medical Products Administration
The Impact of Patent Linkage and Patent Term Extension System on the Industry
Patent Protection of Biological Sequences
Data Supplementation for Chemical and Biotech Patents
IP Challenges and Strategy from Perspective of Innovative Pharma Company under New Environment in China
Hot Issues and Difficulties in Pharma Patent Infringement Litigation Cases
Evaluation of Inventive Step of Pharmaceutical Compound Inventions
Latest Developments of IPR and Recent Patent Invalidation Cases in US
The Strategy of ANDA Filers to Challenge Patents Under a PIV Certification and Recent ANDA Litigation Cases Study
Identifying the Loss of Exclusivity (LoE) of a U.S. Pharmaceutical Patent Portfolio
U.S. Biosimilar Patent Litigation: Strategies and Trends Panel Discussion
IP Issues Related to US and China (Strategy and Challenges)
UPC and UPS - Why does it Make Sense for Europe and What Could be the Impact of Brexit?
Recent European Regulatory Developments on Supplementary Protection Certificates (SPCs)
Biosimilars in US,EU and China: Where Are We Now and Where Are We Going?
How to Build a Successful Licensing Program and Other Strategies for IP Monetization
Patent System and Practice of Medical Fields and Patent Term Extension in Japan
IP Management & Practices Adopted by Generic Drug Manufacturers from India
Patent System for Pharmaceutical Invention in South Korea
Life Sciences: IP and Regulatory Environment in Brazil and selected Latin American Jurisdictions
Current Patenting Situation of Chinese Medicine in China & Analysis from Reexamination and Invalidation Cases
Rethinking of Patent Creative Evaluation for Traditional Chinese Medicine
Relevant Issues on the Protection of Trade Secrets of Traditional Chinese Medicine
IP Challenges and Strategy for Chinese Medicine ‘Going Out’

4th China Pharma IP Summit 2019 (CPIPS2019) will be held on 23rd -25th , Oct this year at Primus Hotel Shanghai Hongqiao China.
As a dedicated event to pharmaceutical intellectual property, In the past three years we gathered more than 1000 attendees from relevant government departments(CNIPA/EPO/JPO/USPTO), industry associations, global and china local pharma/biotech companies, law firms, IP agencies, IP solution providers and so on. Our topics covered the IP protection in all over the world, include mainly China, Europe, US, Japan, South Korea and India. We hope CPIPS could be a good networking and sharing platform for Industry peers, in which we are promoting industry innovation and development.
Part of Confirmed Speakers Snapshots :


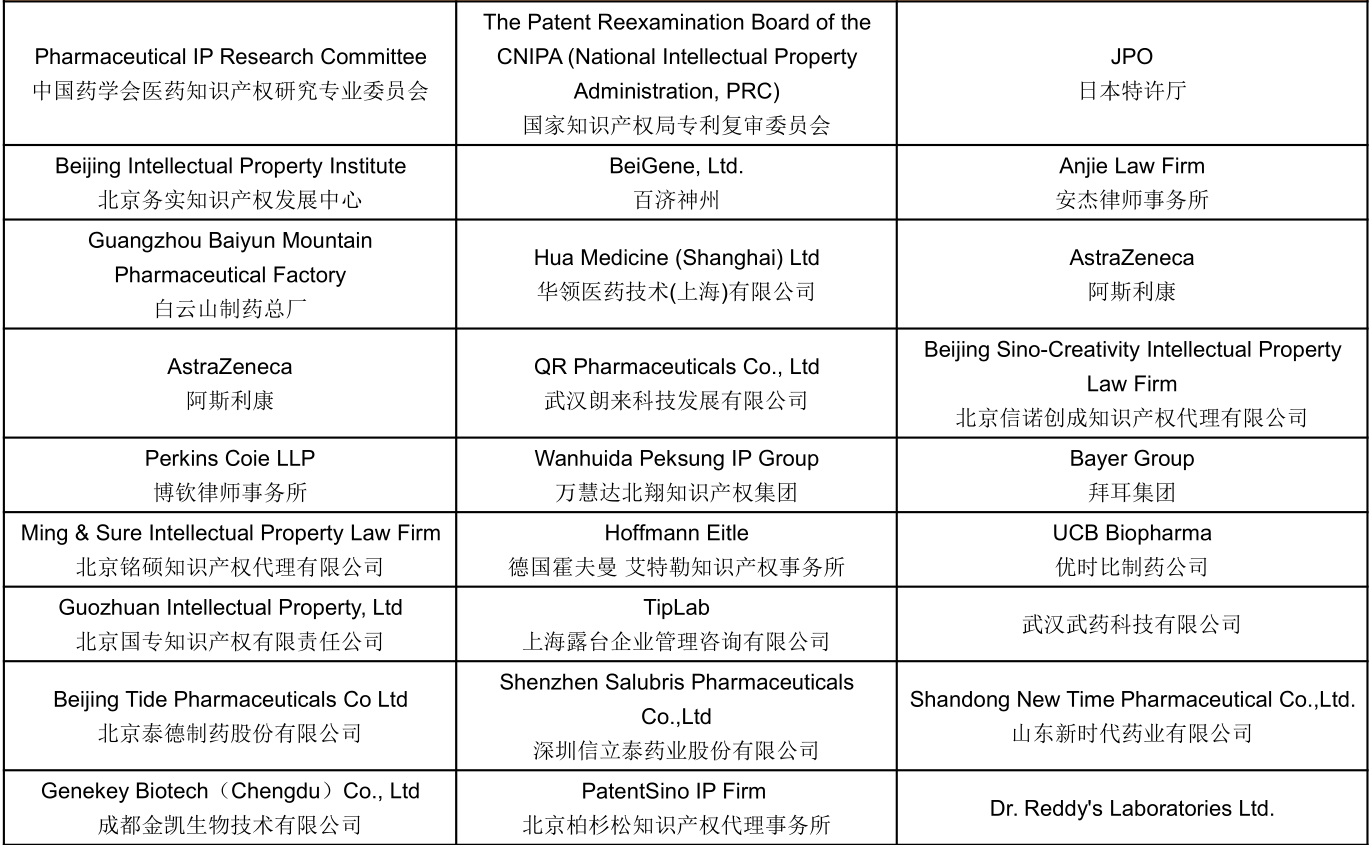

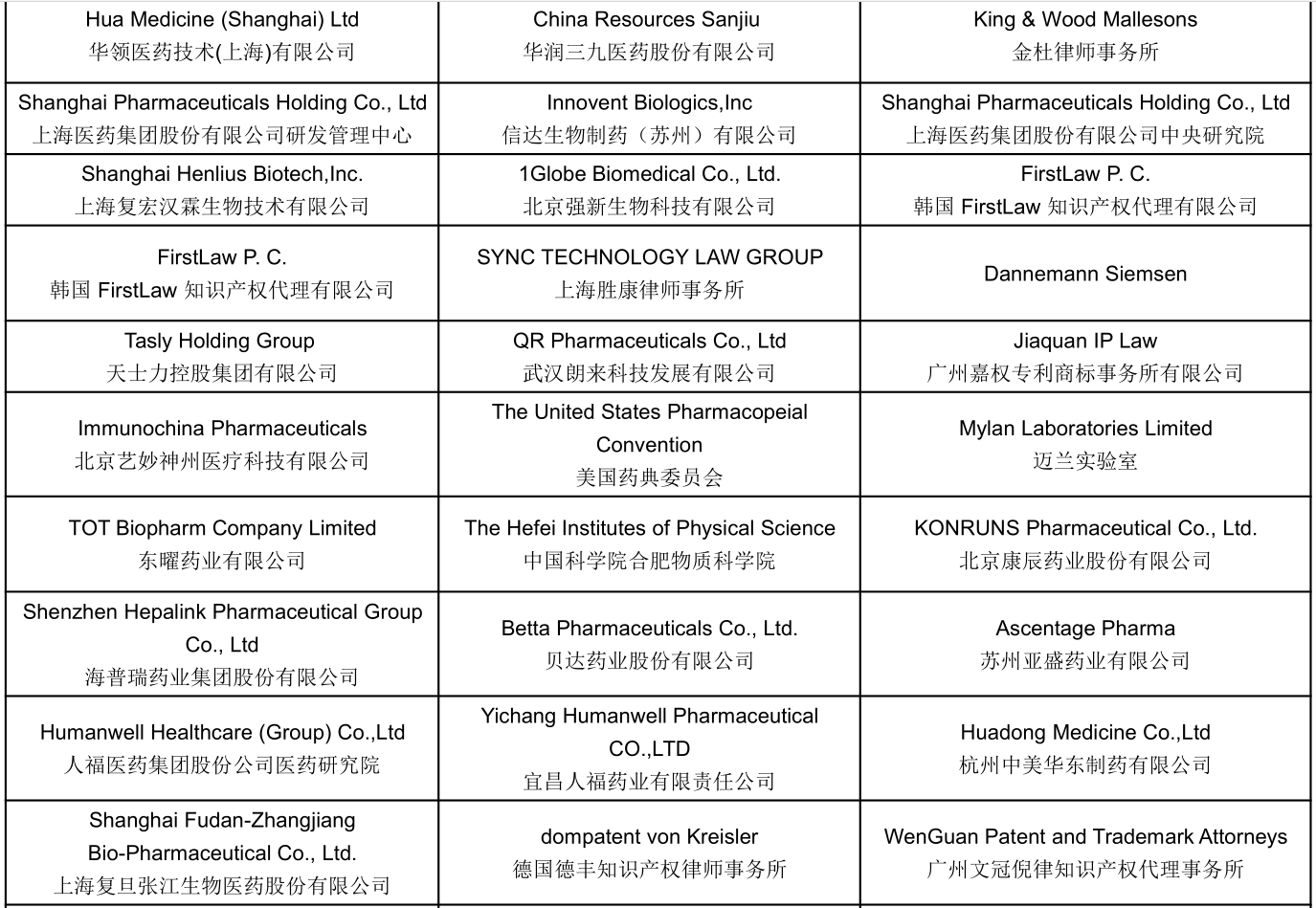
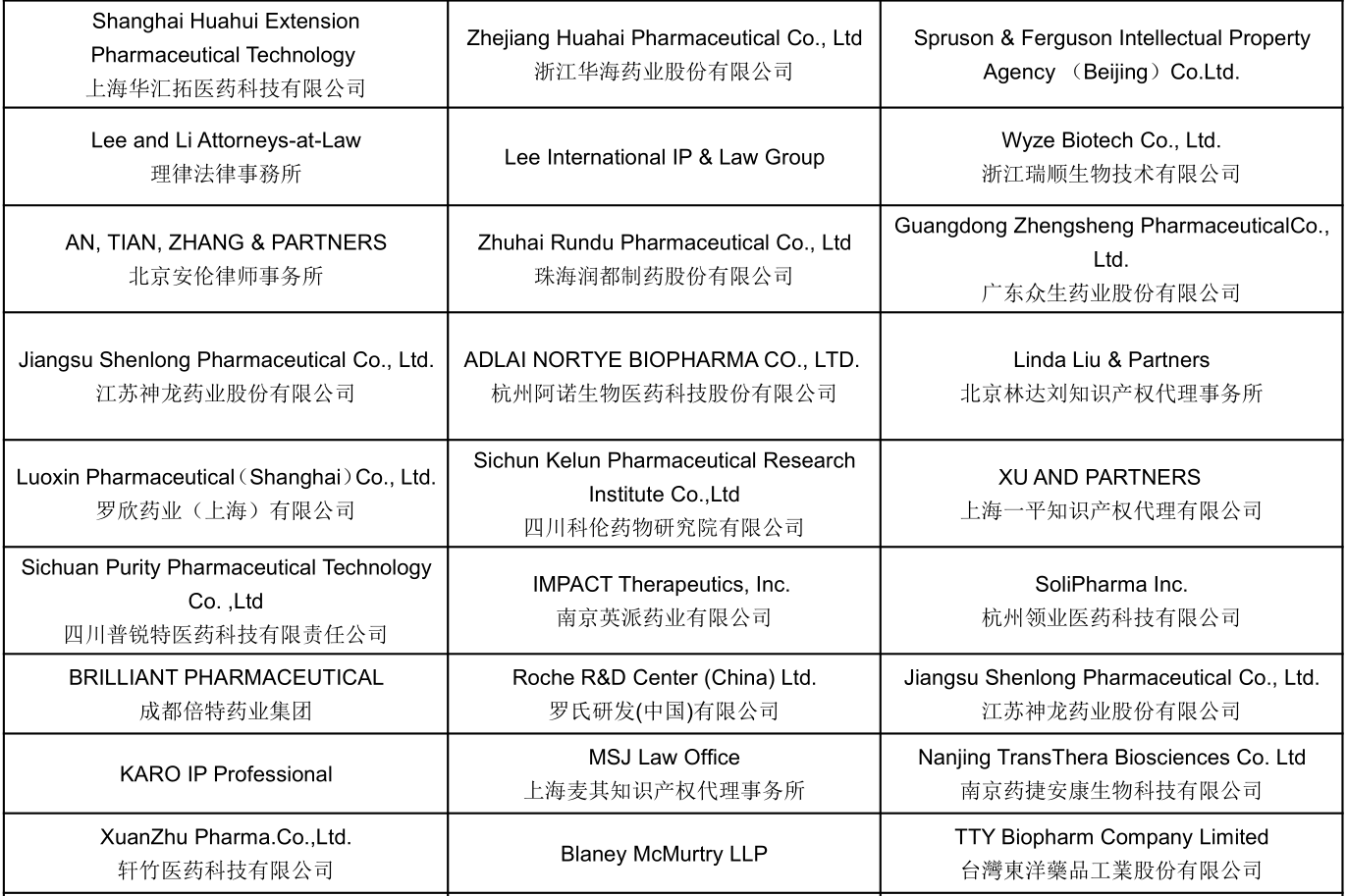
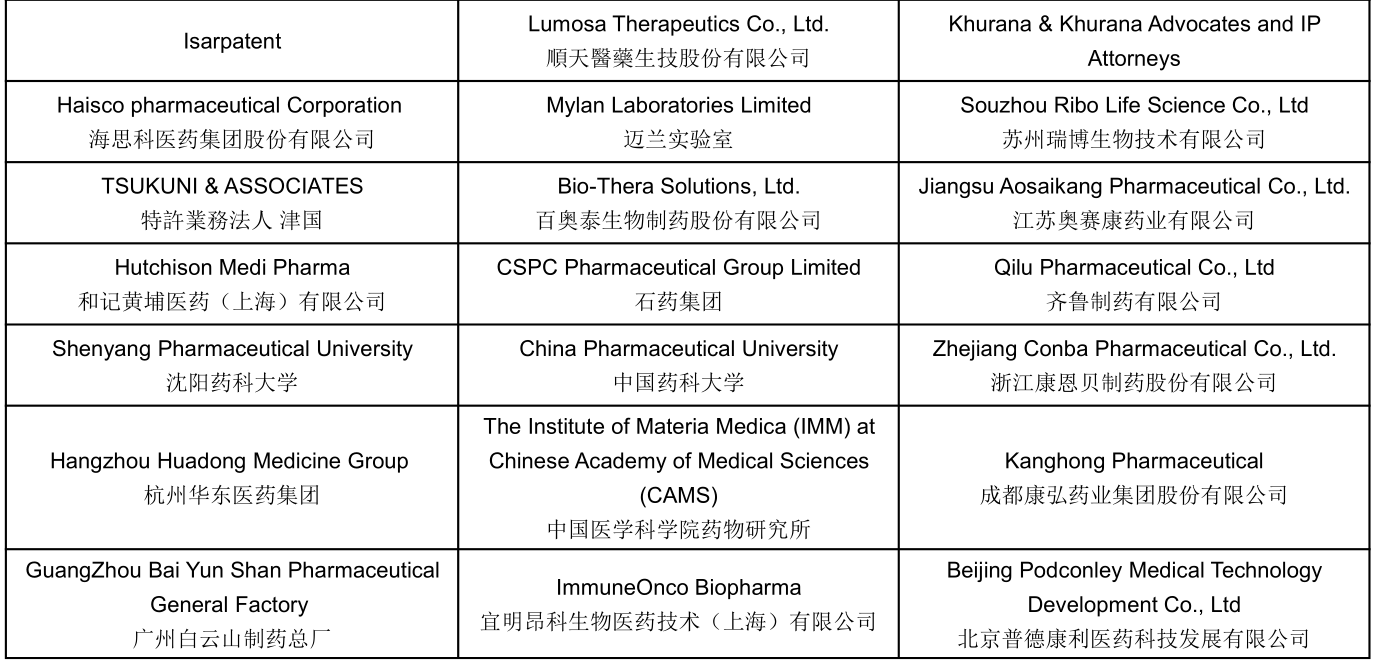

Join US To Meet:
40+ Experts from Government & Authoritative Organizations Give Speeches
200+ Leading China Local & Multinational Pharmaceutical/Biotech Companies
80+ Leading China Local & Foreign Law Firms/IP Agencies
500+ Attendees
Topics Will be Presented and Discussed:
Contact Organizer to get the latest information :
Johnson Li
Conference Manager
Tel: 86 21 6142 1083
Mobile: 86 189 1779 8290
Email: johnson.leung@yipevents.com
Web:www.yipevents.com
Source:IPRdaily(iprdaily.com)
Editor:De
- I also said the two sentence
- Also you can enter 140words






